During my time as a consultant UX designer at Underwriters Laboratories (UL), I partnered with multiple pharmaceutical companies to enhance their design capabilities for medical devices.
My focus was on improving the Instructions for Use (IFU) of combination products, ensuring clarity, usability, and compliance with Health Authority requirements, including FDA submissions. By applying Human Factors Engineering principles, I helped teams create IFUs that support safe and effective product use while meeting regulatory standards.
Boehringer Ingelheim - Respimat Inhaler IFU
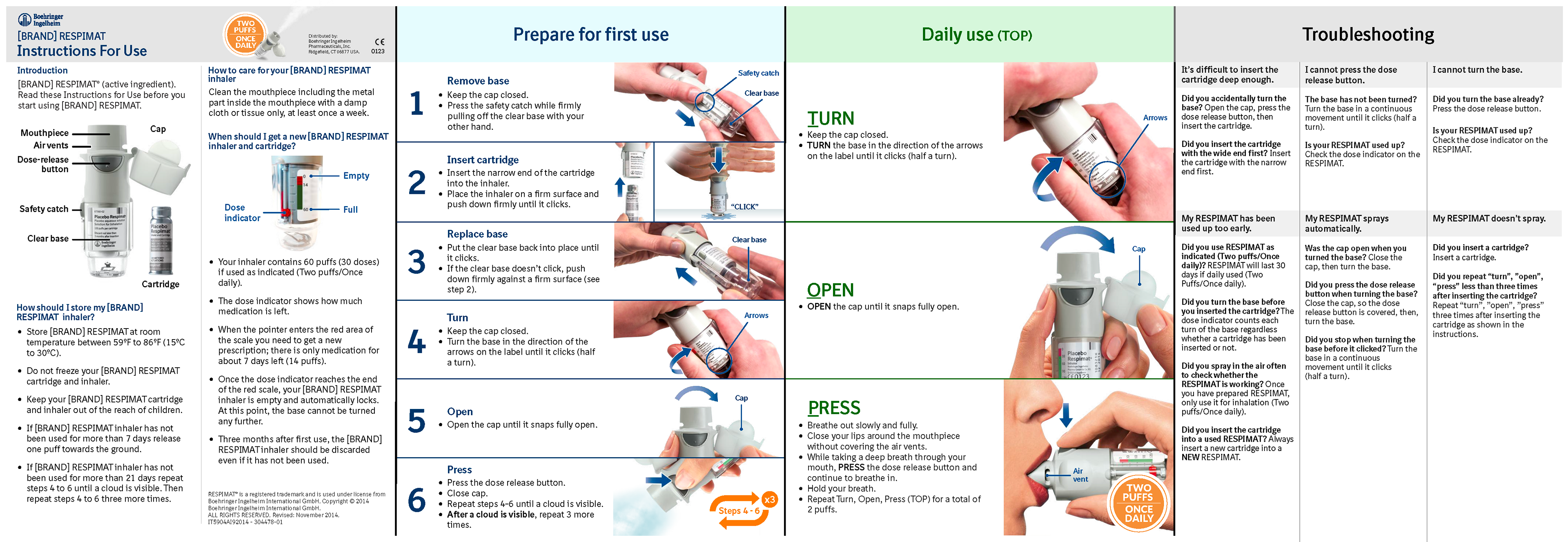
Respimat is for the treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema, and for reducing COPD exacerbations.
Improved layout, color-coded “Prepare” and “Daily use” sections, an improved focus on essential handling steps, use of concise bulleted text, and the incorporation of larger images.
United Therapeutics - Tyvaso Inhalation System IFU
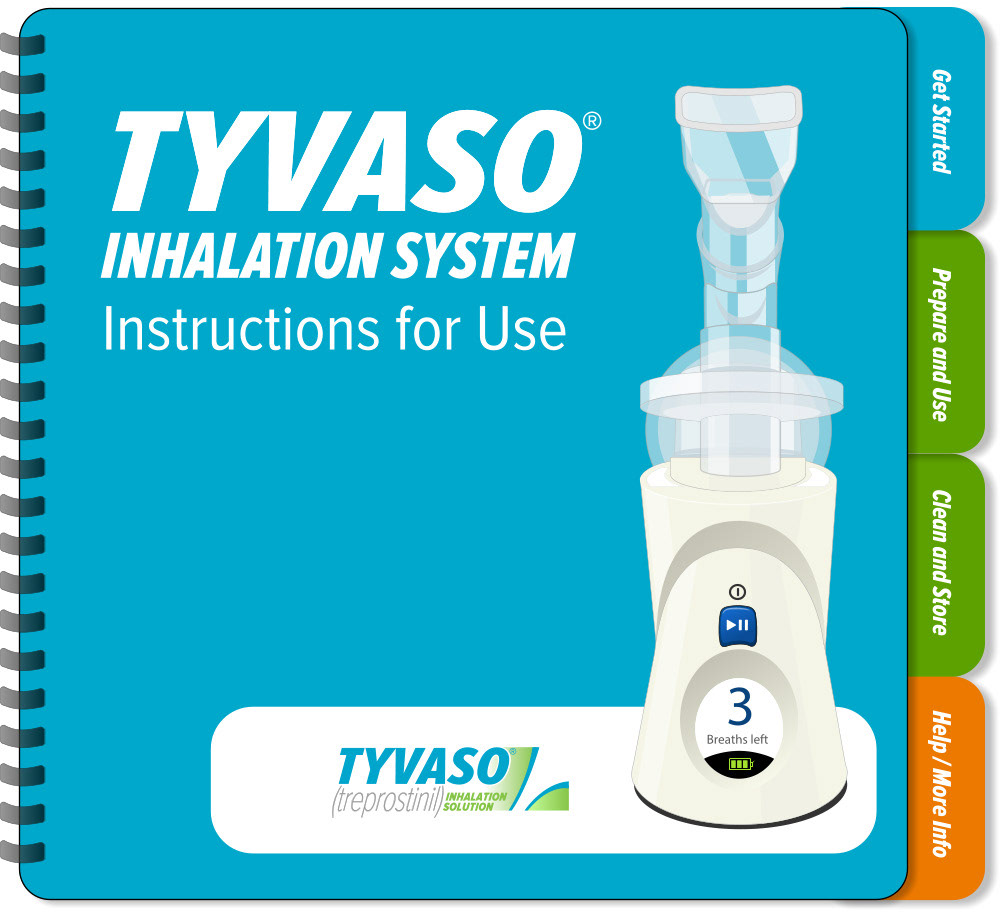
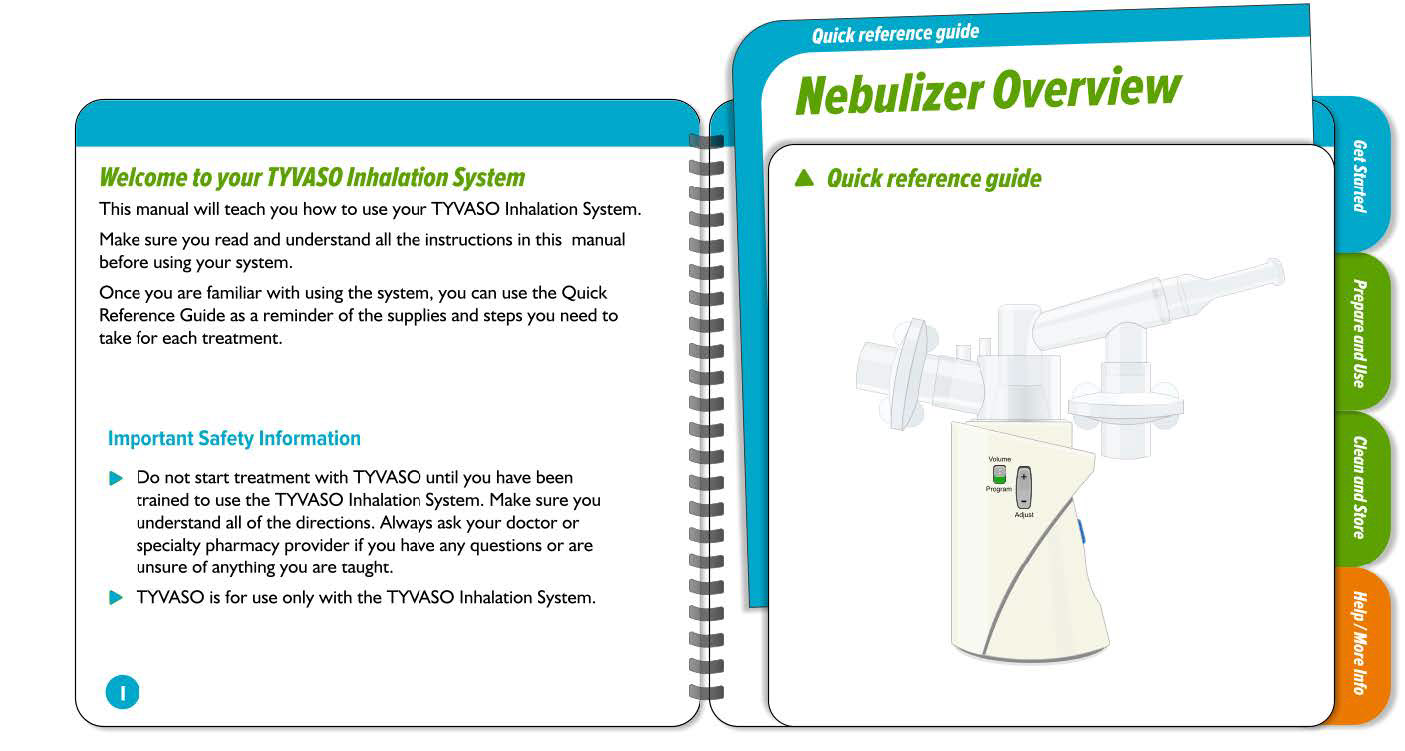
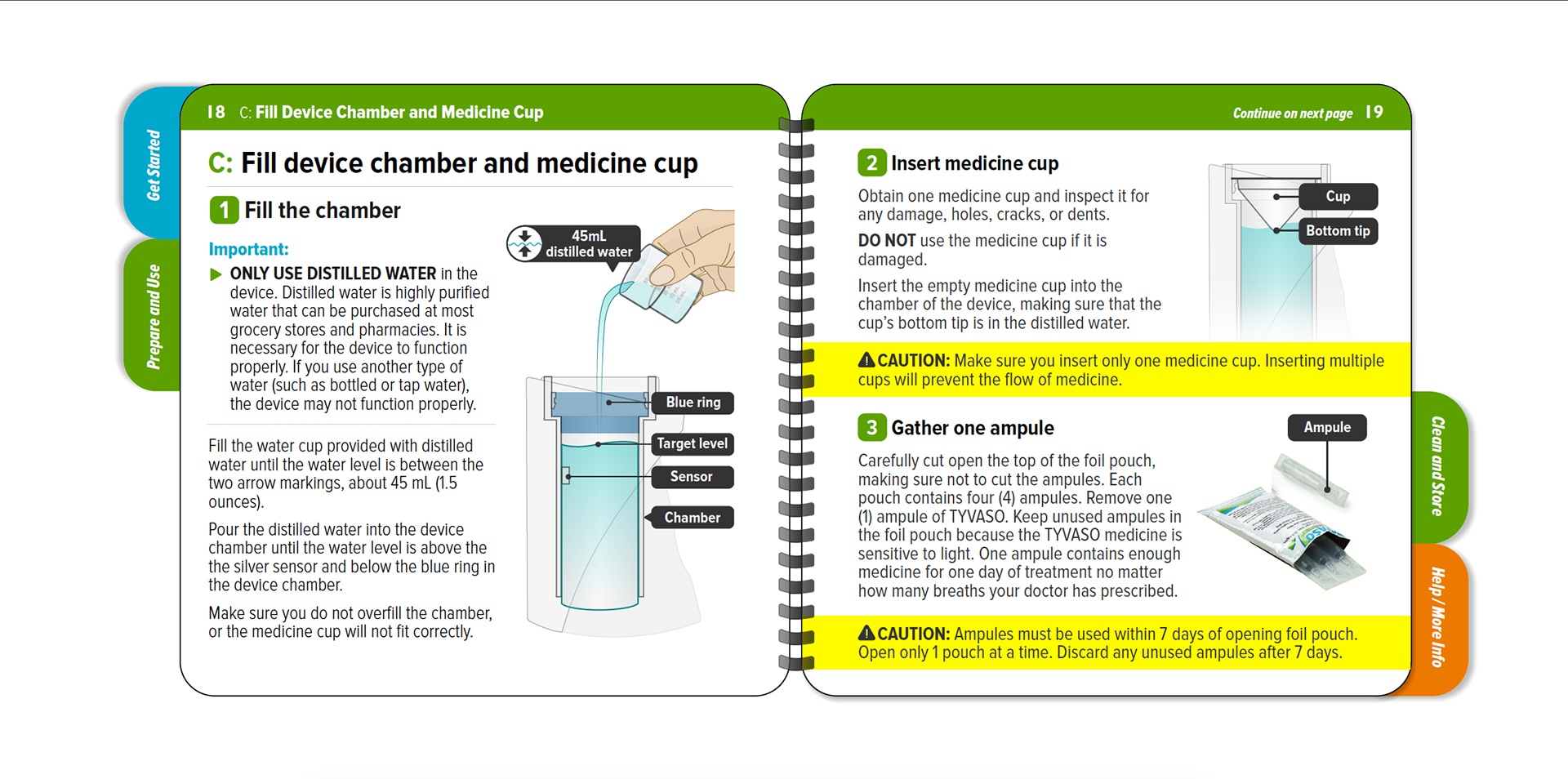
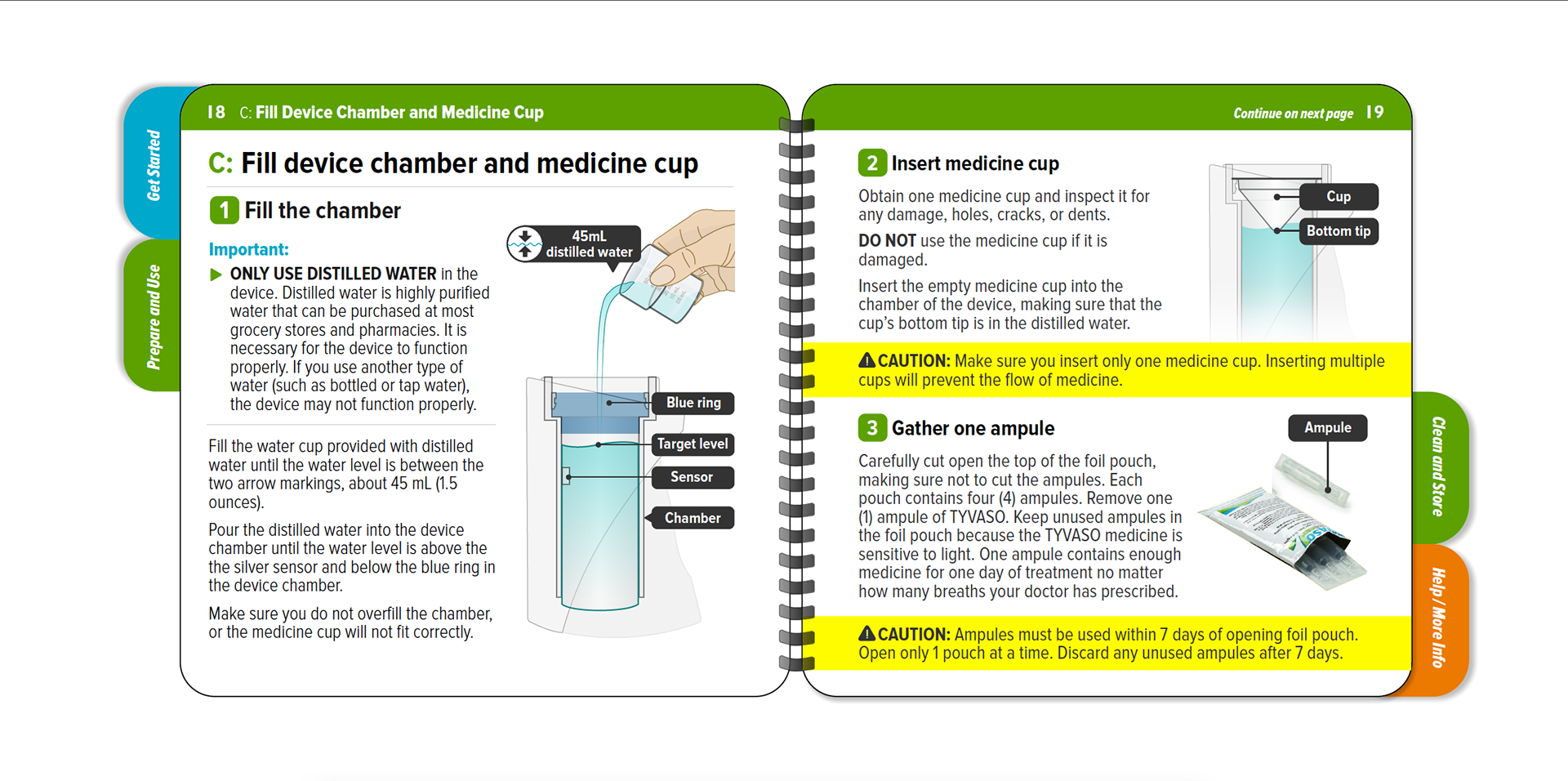
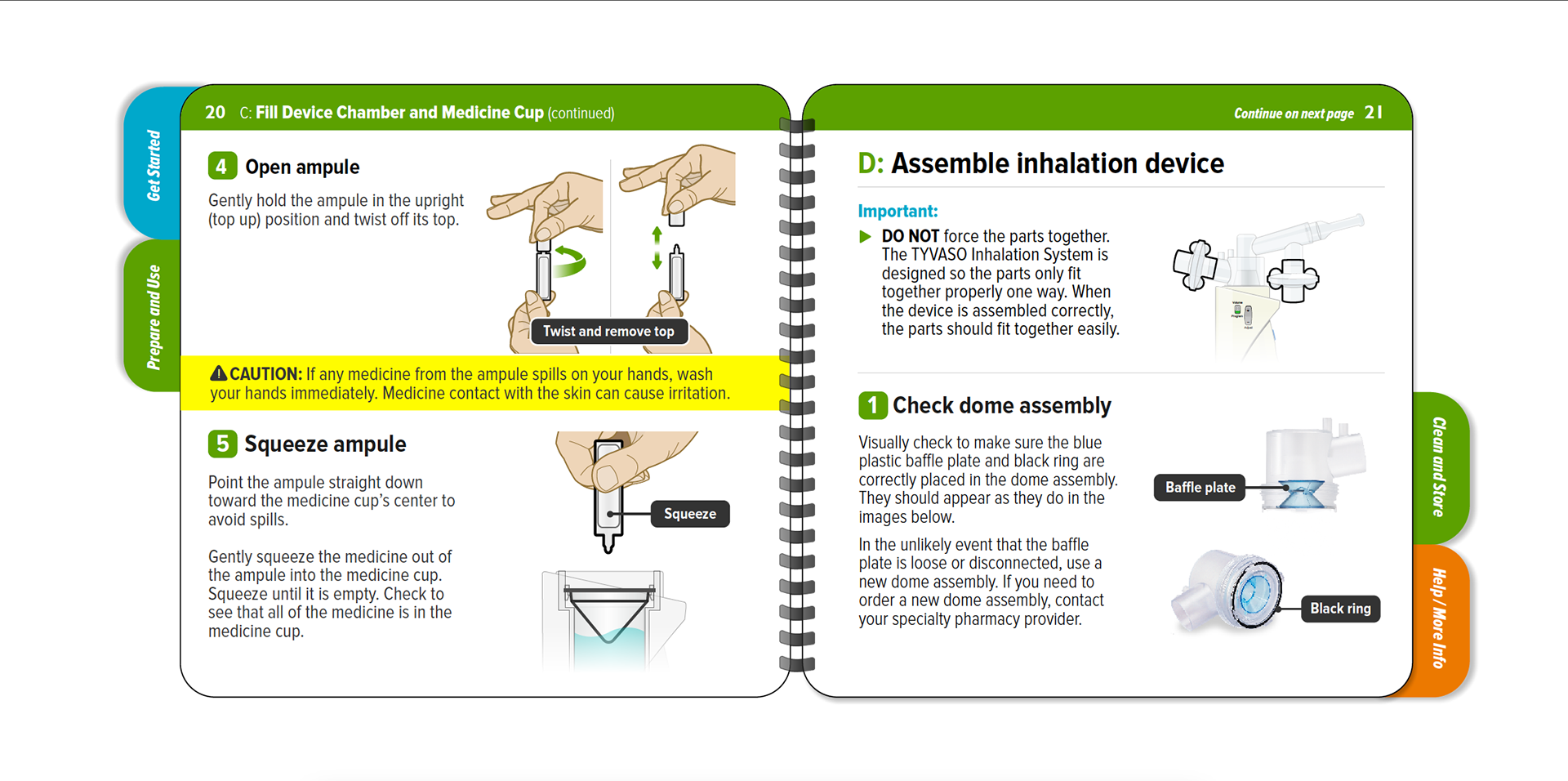
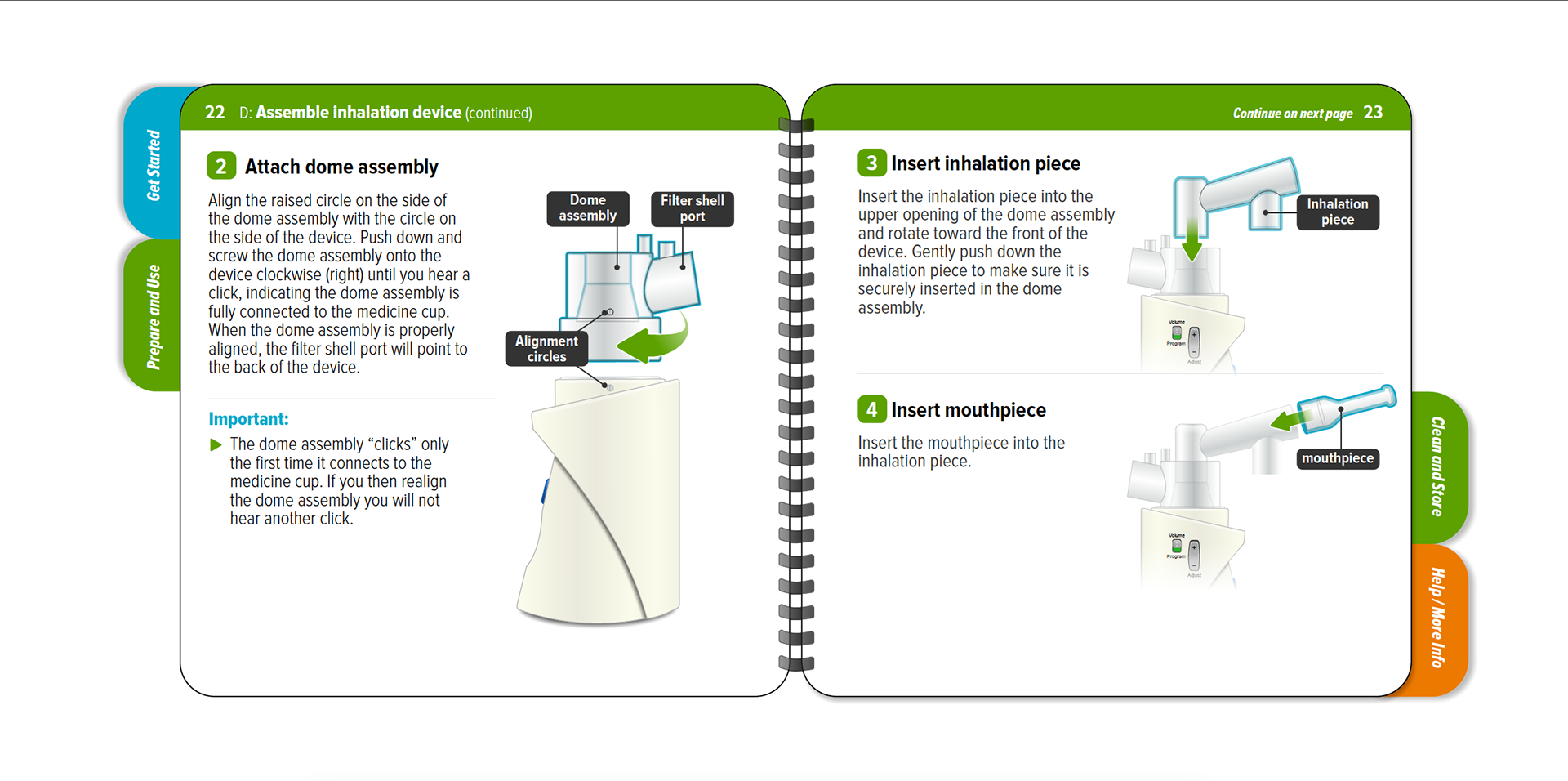
Tyvaso nebulizer is for the treatment of pulmonary arterial hypertension to improve the ability to exercise. A spiral bound IFU, reduced page size, folded QRG, and content organized into four sections to enhance sense of simplicity.
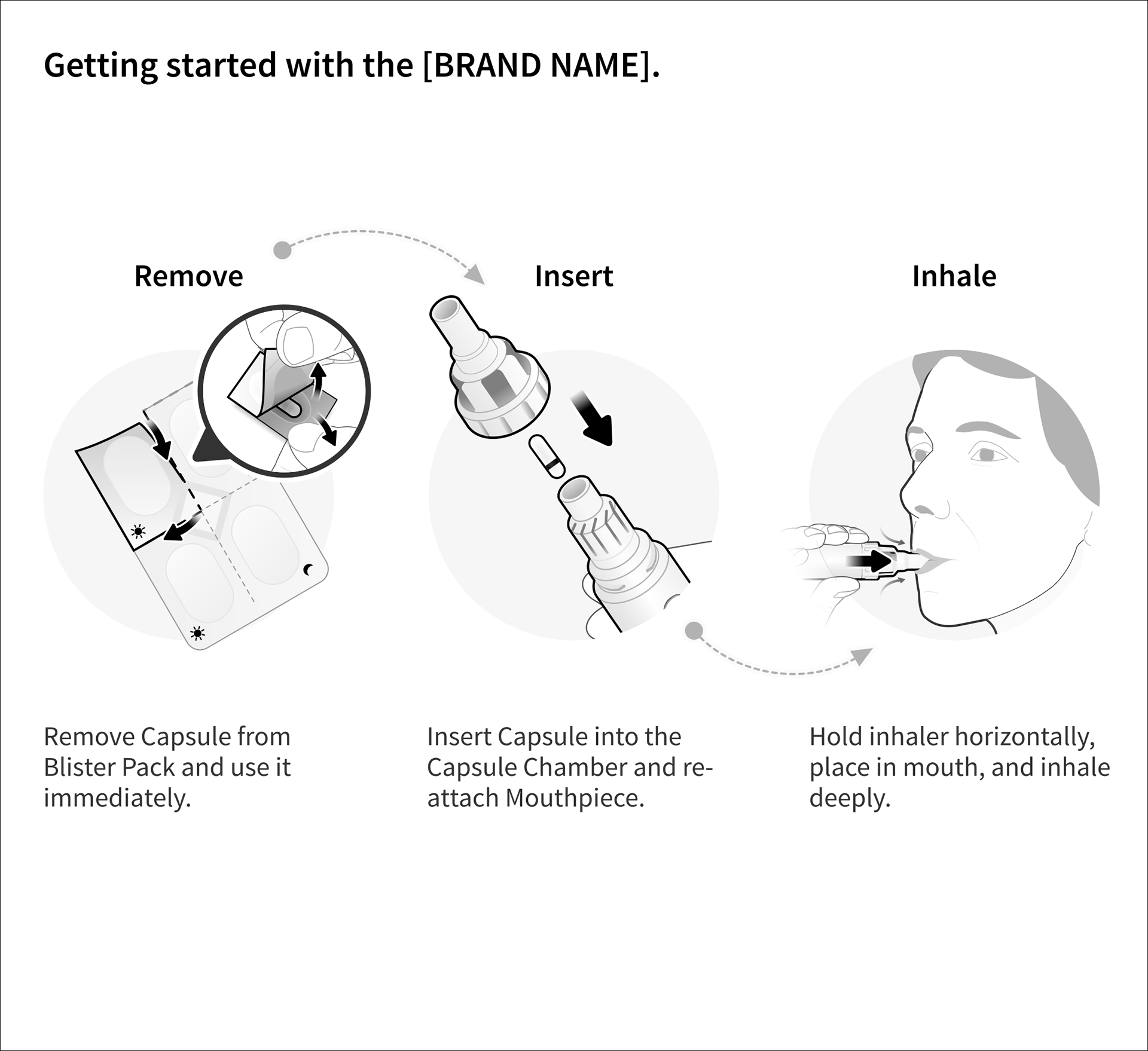
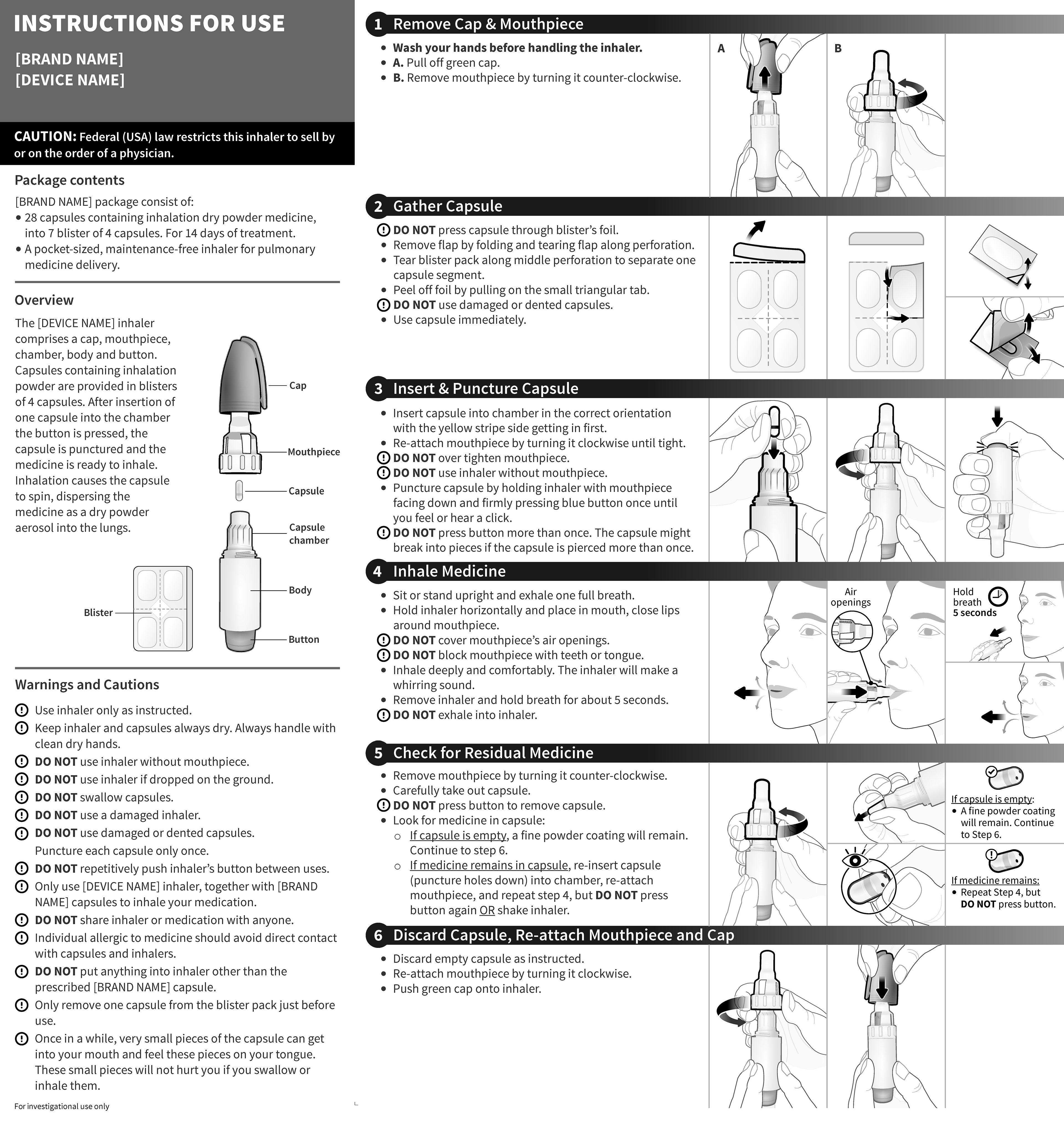
Bayer - Cipro Inhaler QRG and IFU
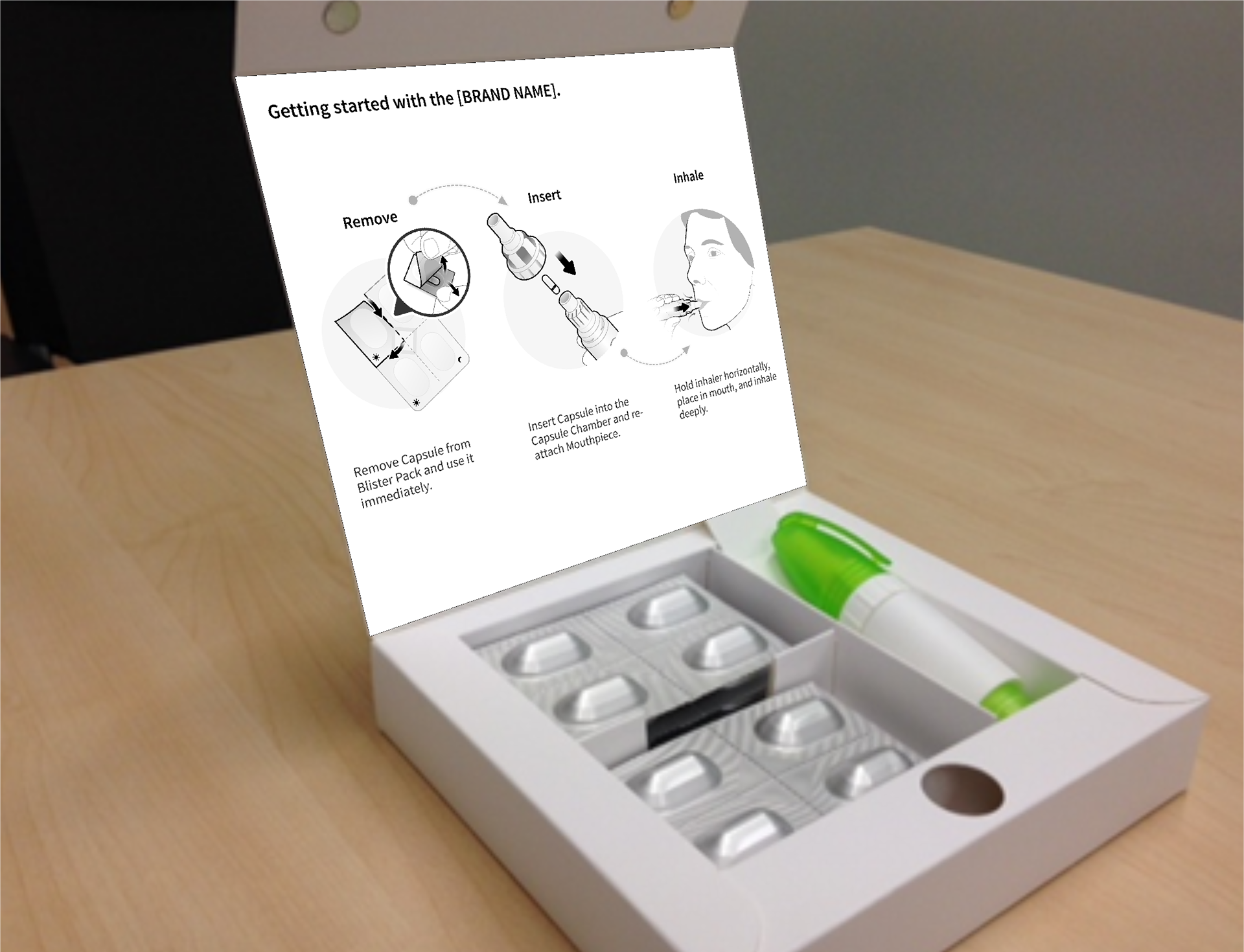
Inner carton panel
Front view
Ciprofloxacin DPI is a dry powder antibiotic device that delivers the medication directly to the site of the infection.
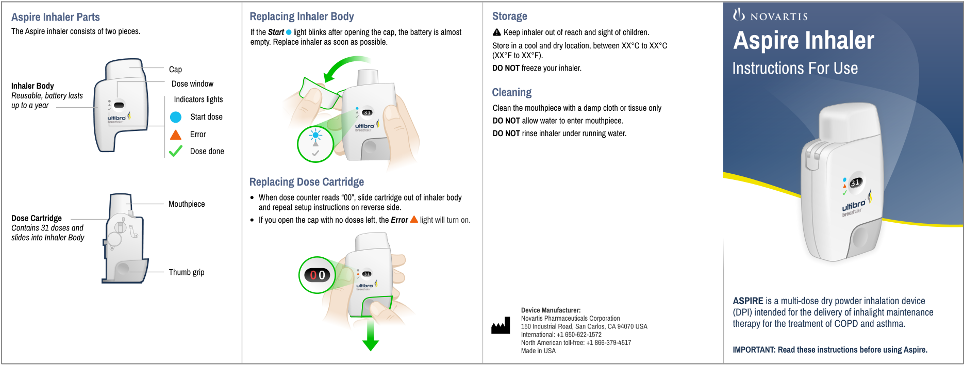
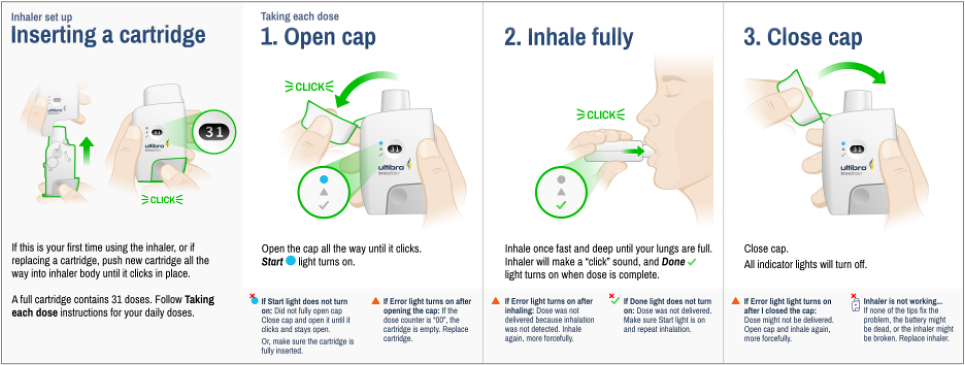
Novartis - Aspire Inhaler IFU
Front view
Back view
Aspire is a multi-dose dry powder inhalation device intended for COPD and asthma therapy and treatment delivery.
Lonhala Magnair - Nebulizer QRG
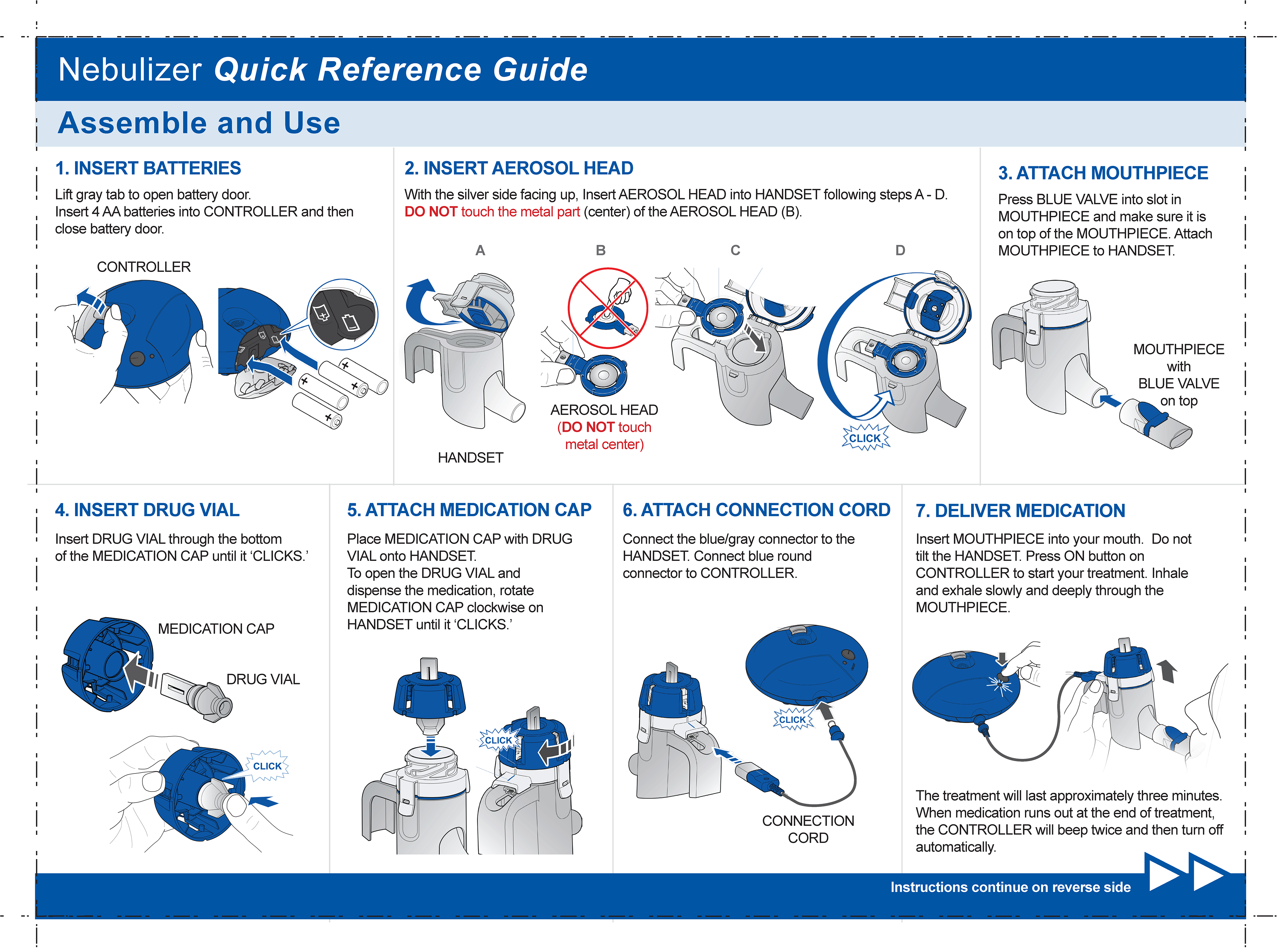
Front view
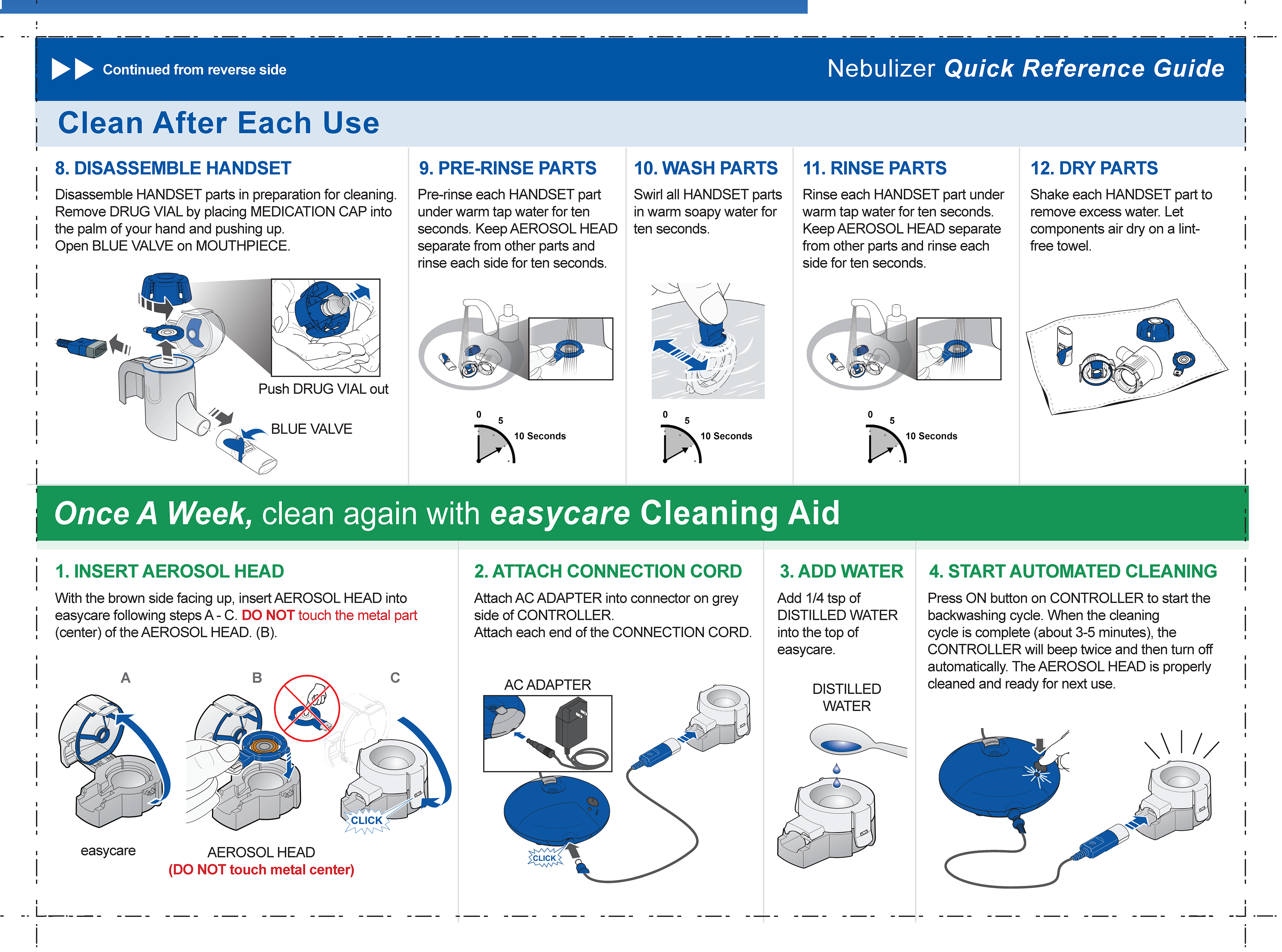
Back view
This is an anticholinergic medicine, used long-term and twice daily to improve the symptoms in adults with COPD.